Hydrogen Production from renewable sources?
0 Introduction
If the hydrogen energy industry wants to develop, it must first solve the problem of sources. At present, according to the way hydrogen is obtained, it can be mainly divided into gray hydrogen, blue hydrogen and green hydrogen. Large-scale hydrogen production basically relies on fossil energy, mainly divided into natural gas reforming hydrogen production and coal gasification hydrogen production, such as these hydrogen production methods, generally known as blue hydrogen. To achieve the whole process of hydrogen energy renewable and clean, it is necessary to use renewable electricity or nuclear energy to produce green hydrogen to achieve.
At this stage, biomass energy, wind energy, solar energy and other renewable energy has developed rapidly, especially solar energy, thanks to the development of technology, the cost of photovoltaic power generation has fallen sharply, and cheap electricity can be obtained through photovoltaic power generation and other ways, so the combination of renewable energy and hydrogen energy, to achieve the whole process of zero carbon emissions become possible. This is also the key to hydrogen as a future clean energy, so the study of hydrogen production from renewable energy is very important for the development of hydrogen energy.
Renewable energy and hydrogen energy combined to achieve zero carbon emissions in the whole process of large-scale hydrogen production mainly by the following ways: electrolytic water hydrogen production (PEM), biomass energy hydrogen production, thermochemical cycle hydrogen production.
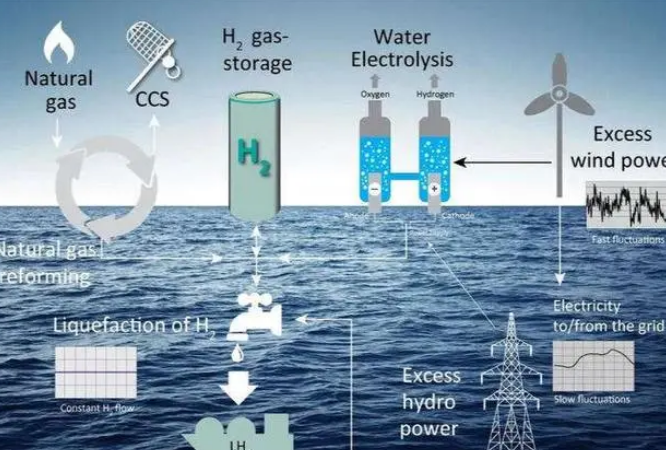
1, electrolysis of water to produce hydrogen
The water electrolysis hydrogen production device generally needs to be composed of water electrolyzer, gas-liquid separator, gas scrubber, electrolyte circulation pump, pressure regulator, measurement and control instrument and power supply equipment.
Hydrogen production by water electrolysis is considered to be the development direction of hydrogen production in the future, especially the use of renewable energy electrolytic water to produce hydrogen, with the potential to transfer a large number of renewable energy electricity to the difficult deep decarbonization of industrial sectors, has become the direction and focus of countries.

Because the efficiency of coal-fired power generation is 30 ~ 45%, the efficiency of electrolytic water is 70 ~ 80%, and the electricity generated by coal-fired power generation is electrolytic water, the overall efficiency of electrolytic water is low, only 20 ~ 35%. Therefore, the use of coal power generation electrolytic water to produce hydrogen is obviously not enough economic and environmental protection.
The principle of electrolytic water hydrogen production is very simple, the theoretical electrolytic voltage of water is 1.23V, so that water can be decomposed, the voltage on the electrode must be greater than the theoretical decomposition voltage of water.
At present, the industrial equipment for electrolyzing water can be divided into: alkaline electrolyzer, solid oxide electrolyzer, polymer film electrolyzer; Electrolytes mainly include alkaline electrolyte and polymer solid electrolyte.
(1) Alkaline electrolyzer: unipolar electrolyzer, bipolar electrolyzer
The single-pole electrolytic cell adopts the mode of parallel electrodes, and the Yin and Yang in the cell are parallel, upright and staggered. Anode and cathode, cathode and cathode are connected in parallel. A gas diaphragm is arranged between the cathode and the anode.
Bipolar electrolytic cell adopts series electrode mode, compact structure, less resistance loss, parallel electrode plate, upright. The electrode is bipolar, one side is a cathode, one side is an anode, and the middle of the two adjacent plates is a diaphragm.
(2) Solid oxide electrolyzer
It can work under high temperature conditions (1000℃), and part of the energy can be provided by heat energy, so the efficiency is high, and the comprehensive waste heat utilization can reach 90%. But the cost is relatively high.
(3) Polymer film electrolyzer (PEM)
PEM water electrolysis hydrogen production technology has the advantage of quick start-stop and can match the volatility of renewable energy generation
Different from alkaline electrolytic water hydrogen production, PEM electrolytic water hydrogen production uses perfluorosulfonic acid proton exchange membrane with good chemical stability, proton conductivity and gas separation as a solid electrolyte to replace asbestos film, which can effectively prevent electron transfer and improve the safety of electrolytic cell. The main components of PEM water electrolyzer are proton exchange membrane, anode and cathode catalytic layer, anode and cathode gas diffusion layer, anode and cathode extreme plate, etc. Among them, the diffusion layer, the catalytic layer and the proton exchange membrane constitute the membrane electrode, which is the main site of the material transmission and electrochemical reaction of the whole water electrolyzer. The characteristics and structure of the membrane electrode directly affect the performance and life of the PEM water electrolyzer. The proton exchange membrane usually consists of a Nafion membrane that transports H+ protons to the cathode.
In addition, compared with the alkaline electrolyzer, the efficiency is low, only 70-80%, the polymer film electrolyzer PEM efficiency can reach more than 85%, the whole equipment is compact, the use of pure water, without alkaline electrolyte, the use of more safe and reliable. In addition, the polymer film electrolyzer PEM can be pressurized during the water electrolysis process, and can directly produce 3-4MPa hydrogen (and the volume fraction of hydrogen can reach more than 99.99%, which is easier to purify to the required purity of fuel cells).
In the past five years, the cost of the electrolytic cell has been reduced by 40%, but the high investment and operating costs are still the main problems to be solved in PEM electrolysis of water hydrogen production, which is closely related to the current oxygen evolution and hydrogen evolution electrocatalyst can only choose precious metal materials. Therefore, reducing the material cost of catalyst and electrolyzer, especially the noble metal load of cathode and anode electrocatalyst, and improving the efficiency and life of electrolyzer are the research focus of the development of PEM electrolysis water hydrogen production technology
2. Hydrogen production from biomass energy
Biomass energy hydrogen production has the following characteristics: mild reaction, low energy consumption, environmental friendliness, wide range of raw materials, renewable and so on. According to the U.S. Department of Energy, microalgae biofuel can replace the fuel of American cars. The quantum efficiency of microalgae converting solar energy is as high as 2-10% (compared to land plants <1%), and the growth rate of microalgae is extremely fast (the daily increase of biomass can reach 1-3 times).
At present, biomass energy hydrogen production is mainly considered from several directions: 1) genetic modification of fermentation hydrogen-producing bacteria to improve hydrogen production efficiency; 2) Hydrogen production through dark and light coupling fermentation; 3) Hydrogen production was increased by pretreatment.
Hydrogen production by fermentation of aquatic biomass such as microalgae has a strong industrial application prospect. In order to promote efficient and low-cost large-scale hydrogen production, some bottleneck problems need to be broken through:
① Promote the hydrolysis and saccharification of biomass waste such as microalgae efficiently and cheaply;
② The hydrogen production efficiency of dark fermentation bacteria was improved by genetic modification and strain domestication;
③ Hydrogen production efficiency and light utilization efficiency of photosynthetic fermentation of biomass were improved by reactor design and strain;
(4) Optimization of heat and mass transfer flow and coupling conditions in a continuous flow reactor coupled with dark light.
3, thermochemical cycle decomposition of water to produce hydrogen
The thermochemical cycle of splitting water to produce hydrogen is the process of using heat energy to split water into hydrogen and oxygen through a series of different but interrelated chemical reactions.
The thermochemical cycle decomposition of water for hydrogen production has the following obvious advantages: 1) low energy consumption (compared with electrolytic water and direct pyrolysis water, low cost); 2) It is easier to realize industrialization (mild reaction); 3) Can directly use the heat energy of the reactor, save the step of power generation, high efficiency; 4) In the thermochemical cycle, hydrogen and oxygen are usually produced in different reaction steps, so there is no need for high-temperature hydrogen-oxygen separation.
The thermochemical cycle decomposition water to produce hydrogen is usually evaluated by the cycle efficiency E (cycle efficiency E: the ratio of the high calorific value of hydrogen (285.8kJ/mol) to the total heat energy required by the cycle). In addition, other evaluation indicators: thermal efficiency, number of cyclic chemical reactions, side reactions, reactant toxicity, reactant price, reactant separation, corrosion problems, raw material handling problems, maximum reaction temperature and thermal conversion problems.
Thermochemical cycle reaction to produce hydrogen was originally proposed by J.E.Funk of the University of Kentucky in 1960, and its principle can be expressed by the following general formula of chemical reaction:
A and B in the formula are called cyclic reagents. The step heating method is used to make the reaction continue to cycle, so as to achieve the purpose of continuous hydrogen production.
In the early 1970s, McKeady and Beni proposed the Mark1 thermal-chemical hydrogen production scheme, and estimated that the efficiency could reach about 55%. The cycle first studied by Marcheltti and DeBeni at Ispra in Italy, called the Mark1 cycle, belongs to the halide system, but it is not suitable for large-scale industrial applications due to the highly toxic Hg involved in this cycle system. People later proposed that circulation systems such as Mark2, Mark3 and Mark6 had replaced the use of Hg, but the whole system was still too complex and not suitable for large-scale industrial application.)
Subsequently, Italy, Germany, the United States, Japan and other countries began to invest a lot of research in thermochemical cycle hydrogen production, and there are hundreds of thermochemical hydrogen production cycles. Among them, the more promising thermochemical recycling methods for hydrogen production are mainly based on the four-step thermal-chemical hydrogen production cycle (UT-3 cycle) of calcium-bromo-iron compounds and the three-step thermal-chemical hydrogen production cycle (SI cycle) based on sulfur-iodine compounds proposed by Kameyama and Yoshida et al., University of Tokyo, Japan. The sulfur iodine cycle is the most studied and most promising cycle in all the thermochemical hydrogen production so far.
In addition to meeting the temperature requirements in the process of choosing thermo chemical hydrogen production cycle, the following problems should be paid attention to: 1) the yield of each step reaction must be high, and the reaction steps should be as few as possible; 2) Circulating reagents A and B should be cheap and readily available in the existing industry; 3) The intermediate products of each step of the reaction are easy to deal with and do not produce by-products, and the processing of intermediate products will increase the complexity of the system and lead to an increase in cost; 4) The whole process will not cause any impact on the external environment, such as pollution.

4. Economic analysis of hydrogen production from renewable energy sources
Taking hydrogen production by electrolysis as an example, hydrogen production by electrolysis is the core link of the future renewable energy power generation system. In order to achieve large-scale green hydrogen energy storage and application, the cost of hydrogen production by electrolysis, as an important limiting factor, must be further reduced.
Using the Levelized Cost of Hydrogen (LCOH) formula, it is estimated that the electrolytic efficiency of 1MW alkaline water electrolytic hydrogen production system can reach 54.88kW·h/kg, equivalent to 4.9kW·h of 1m3(standard) hydrogen. The levelized cost (LCOH) of electrolytic hydrogen production is $4.2 /kg, and from the point of view of the price composition, electricity accounts for about 75% of the cost of hydrogen production. At the same scale, due to the use of rare precious metals Ir and Pt as catalysts for PEM electrolytic reactors, the fixed cost and variable operation and maintenance cost are high. Although the electrolytic efficiency of PEM (49.28kW·h/kg) is better than that of alkaline electrolytic water system, the levelized hydrogen cost of PEM electrolytic hydrogen production is about 20% higher than that of alkaline electrolytic water system. It is not yet economical. Combined with the on-grid electricity price of wind power and photovoltaic power stations announced by the National Development and Reform Commission in 2019, assuming that the on-grid electricity price of renewable energy power generation system is 0.40 yuan /(kW· h), it can be roughly calculated that the cost of hydrogen production by alkaline water electrolysis is 2.61 yuan /m3(standard), which is converted into an equivalent unit calorific value price of 0.206 yuan /MJ. Natural gas is calculated according to 4 yuan /m3(standard), and the equivalent unit heat value price of natural gas is 0.1 yuan /MJ. Therefore, if the electrolytic hydrogen is simply priced by heat, the hydrogen energy of the renewable energy power generation system is not economically enough for external sale in terms of hydrogen power generation and urban gas mixed with hydrogen for gas supply.
However, if high-quality hydrogen is sold for industrial production and transportation, the external sales price of industrial hydrogen is between 2 and 3 yuan /m3(standard), so that the electrolytic hydrogen production of renewable energy power generation system has a certain economy. Between 2010 and 2019, the power generation cost of photovoltaic and onshore wind power decreased by 82% and 39% respectively, and there is still a large room for decline in the future, when the cost of electricity is reduced to 0.1 yuan /(kW·h), the cost of electrolytic hydrogen production is equivalent to that of coal hydrogen production, about 12 yuan /kg. If the carbon tax is included, a carbon tax of 100 yuan /t will increase the cost of coal to hydrogen by about 6 yuan /kg.
5. Conclusions and Suggestions
According to the classification of carbon emissions in the hydrogen production process, hydrogen is divided into gray hydrogen, blue hydrogen and green hydrogen. In order to comply with the carbon emission reduction policy, the renewable energy hydrogen production method belonging to green hydrogen will be the mainstream way of hydrogen production in the future. There are a variety of ways to produce hydrogen from renewable energy sources, and the following is a list of several key points to pay attention to in the process of choosing hydrogen from renewable energy sources.

① Carbon emissions. The primary energy input for hydrogen production should all come from renewable energy sources (wind energy, solar energy, etc.), and the process of hydrogen production needs to consider the process carbon emissions, and the total carbon emissions should at least meet the green hydrogen standard (less than 4.9kg CO2e/kg H2).
② Cost. At present, the mainstream method for obtaining hydrogen is industrial by-product hydrogen, because it is simple to obtain, and the cost is low, only need to purify the gas, but the capacity is limited. For renewable hydrogen production to be applied on a large scale, its cost must be comparable to or lower than the current cheaper natural gas reforming hydrogen production.
③ Scale. The hydrogen production method should be reproducible and have the conditions for large-scale production. If you want to use hydrogen energy as a means of peak regulation of the grid, then the hydrogen production capacity can at least reach the level of absorbing excess solar, wind and other intermittent renewable energy sources.
④ High efficiency. Hydrogen production methods need to consider the issue of efficiency, hydrogen production efficiency is too low will not only lead to waste a lot of primary renewable energy, in a certain amount of hydrogen production projects will also need more construction resources, thus hindering scale.
⑤ Do not occupy a certain amount of necessary resources. In the future, the social energy system will be built around electrification, and if hydrogen energy is used as the carrier of energy and the way of energy storage to cooperate with renewable energy, there will be a process of electricity to hydrogen and hydrogen to electricity. At this time, hydrogen production can only be used as a regulatory means to deal with intermittent and surplus renewable energy, store the surplus intermittent energy, and finally convert hydrogen energy into electricity when there is a shortage, and stable renewable energy will not be the energy source target for hydrogen production.
From the above points of view, under the current conditions, wind power and photovoltaic power generation hydrogen production is the best renewable energy hydrogen production method, and there have been successful demonstration examples at home and abroad. Hydrogen production from other renewable energy sources is more or less faced with large-scale, low efficiency problems, and does not have application prospects. However, hydrogen production from wind power and photovoltaic power generation is also facing cost problems, but the cost can be reduced through technological progress.
- ABB
- General Electric
- EMERSON
- Honeywell
- HIMA
- ALSTOM
- Rolls-Royce
- MOTOROLA
- Rockwell
- Siemens
- Woodward
- YOKOGAWA
- FOXBORO
- KOLLMORGEN
- MOOG
- KB
- YAMAHA
- BENDER
- TEKTRONIX
- Westinghouse
- AMAT
- AB
- XYCOM
- Yaskawa
- B&R
- Schneider
- Kongsberg
- NI
- WATLOW
- ProSoft
- SEW
- ADVANCED
- Reliance
- TRICONEX
- METSO
- MAN
- Advantest
- STUDER
- KONGSBERG
- DANAHER MOTION
- Bently
- Galil
- EATON
- MOLEX
- DEIF
- B&W
- ZYGO
- Aerotech
- DANFOSS
- Beijer
- Moxa
- Rexroth
- Johnson
- WAGO
- TOSHIBA
- BMCM
- SMC
- HITACHI
- HIRSCHMANN
- Application field
- XP POWER
- CTI
- TRICON
- STOBER
- Thinklogical
- Horner Automation
- Meggitt
- Fanuc
- Baldor
- SHINKAWA
- Other Brands




































































































































