GE MDS GPA-9 power amplifier
GE MDS GPA-9 power amplifier
DESCRIPTION
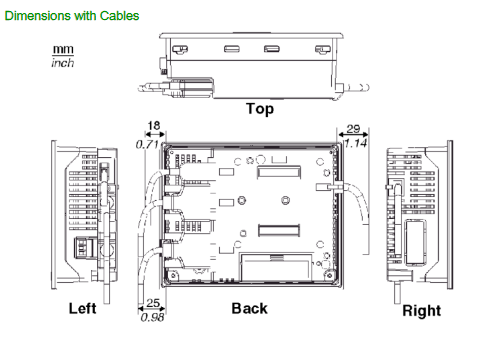
The GPA-9 power amplifier consists of an RF amplifier and PCB mounted to a heat sink, with a DC Power interface, PTT and input/output RF connections on the sidewalls of the chassis. DC power is supplied to the amplifier from a regulated and filtered DC source capable of supplying 10-16 Vdc at a maximum current of 10 Amperes. The DC power source should be current limited or have a protective fuse or circuit breaker.
Unit Specifications
Operating Voltage:10-16 Vdc. 13.8V nominal
Maximum Current Draw:10 Amperes @ 13.8V, 40W RF Out.
RF Out:+40 to +46 dBm (10-40 watts), adjustable
RF Drive Power:SD9 PWR=30dBm
Duty Cycle (GPA-9A model):100% up to full output power
Duty Cycle (GPA-9B model):30% above 10 Watts
Operating Frequency:896-940 MHz
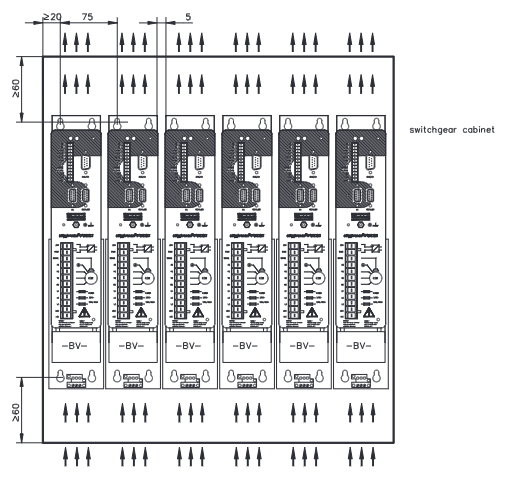
Mounting:standard 19-inch rack cabinet

Power Amplifier Overview
A power amplifier (Power Amplifier) is an electronic device used to amplify the power of an input signal and then output it, usually used to drive loads such as speakers and antennas. It is widely used in many fields such as medical, industrial, communication and audio.
Possible technical features of GE MDS GPA-9
Although it is impossible to directly obtain the detailed technical parameters of GE MDS GPA-9 Power Amplifier, based on the general characteristics of power amplifiers and GE's background as a medical equipment manufacturer, we can reasonably assume that this model may have some of the following technical characteristics:
High Efficiency: As a key component in medical devices, the GPA-9 may have adopted highly efficient power amplification technology to ensure that it provides sufficient output power while maintaining low energy consumption and heat generation.
Stability: Medical devices have extremely high requirements for stability and reliability. Therefore, GPA-9 may adopt advanced circuit design and protection measures to ensure stable operation under various working environments and reduce the failure rate.
Precise control: In applications such as medical imaging, where precise power control is critical, the GPA-9 may have precise power regulation to meet the needs of different application scenarios.
Compatibility: For seamless integration with other GE medical devices, the GPA-9 may feature standardised interfaces and communication protocols to ensure compatibility with existing systems.
Safety: The safety of medical devices is a top priority, and the GPA-9 may feature multiple safety protection mechanisms, such as overload protection and short-circuit protection, to ensure that abnormal conditions do not cause damage to equipment and personnel.
Instructions and warnings related to the operation of the product:
The following specifications must be strictly observed:
The technical specifications and typical applications of the product system must be strictly observed.
PERSONNEL TRAINING: Only trained personnel may install, operate, maintain or repair the product system. These personnel must be instructed on the
These personnel must be instructed and briefed on the conditions in the hazardous area.
Unauthorised modifications: No modifications or structural changes may be made to the product system.
Maintenance Responsibility: It must be ensured that the product system is only used under appropriate conditions and in a condition that is fully fit for purpose.
Working environment: The user must fulfil the specified environmental conditions:
Safety regulations
The following safety regulations must be fully observed when (maintenance) work is carried out on the product system:
1 Disconnect completely.
2 Secure to prevent reconnection.
3 Confirm that the installation has been completed.
4 Perform grounding and short-circuiting.
- User name Member Level Quantity Specification Purchase Date
- Satisfaction :
-