Dead leaves, human waste and other biomass can actually generate electricity?
In SOFC, fuel oxidation occurs in the anode chamber. Oxygen is ionized in the cathode chamber and moves through the electrolyte to the anode chamber. In the anode chamber, the gas fuel is dispersed at the interface of the electrolyte and an electrochemical catalytic reaction occurs. Electrons in the fuel molecules travel through an external circuit to the other pole, generating electricity. The cathode receives electrons through an external circuit, reducing oxygen molecules and forming oxygen ions at the interface of the solid electrolyte. Oxygen ions move through the solid electrolyte to the anode and form either H2O or CO2, depending on the fuel type. The properties of the fuel cell electrolyte determine the operating temperature of the battery. In SOFC, the operating temperature is very high, close to the temperature of the gasification process. The higher operating temperature of SOFC makes it possible to combine biomass gasification technology with SOFC to improve power generation efficiency.
In recent years, there has been a focus on optimizing biomass gasification conditions and eliminating problems that arise during this process, including the formation of impurities such as ash, tar and other alkaline complexes. Compared with oxygen and air gasification, biomass vapor gasification coupled with SOFC can achieve maximum power generation efficiency. The entire energy conversion process has several performance indicators, including thermodynamic conversion efficiency, capital and operating costs, and environmental impact. The next step of research is to overcome the difficulty of material selection due to the properties of the materials, with the expectation that the properties of these materials can be clearly understood, thereby maximizing the power generation.
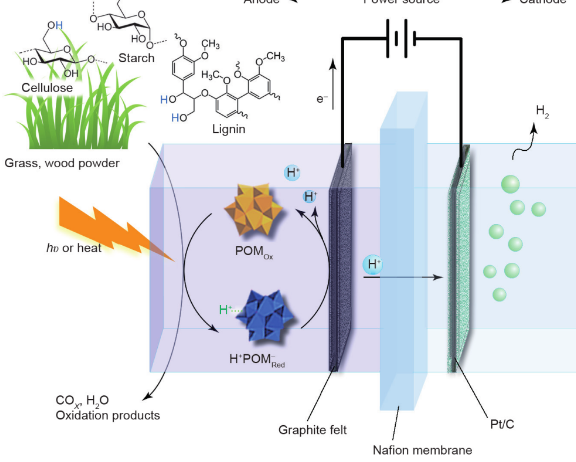
DBFC technology has recently been reported in the literature. This technology generates electricity directly from biomass without the need to pretreat or process the biomass to obtain liquid or gaseous fuel. In DBFC, different forms of lignocellulosic biomass, such as wood, grass, agricultural waste, algae, etc., can be converted into electricity. However, currently available DBFC technologies can only utilize refined biomass, such as starch or cellulose. There are still many shortcomings and challenges to be addressed in developing advanced power generation technologies that use fuel cells to convert biomass directly into electricity.
(3) Microbial fuel cells
In order to overcome the difficulties presented by traditional technologies, MFC has gained the attention of the scientific community in the past 10 years. This type of fuel cell can generate electricity from organic biomass. MFC is a heterogeneous reactor that uses microorganisms to convert organic compounds into electrical energy at low temperatures. In lignocellulosic biomass-based MFC, the biomass is first converted into fuels in the form of glucose, xylose, acetate, etc., for further oxidation reactions by microorganisms. The MFC consists of two electrolytic chambers, where two electrodes are separated by a semi-permeable membrane (proton exchange membrane (PEM) or anion exchange membrane (AEM)), and an external circuit. Microbial oxidation of fuel in anode chamber, typical products are CO2, protons and electrons. The electrons are captured by the anode and enter the cathode through the external circuit, while the protons on the anode enter the cathode through the membrane, and combine with the oxygen in the cathode and the electrons in the external circuit to form water. The potential difference between the cathode and the anode produces electrical energy. The microbes act as a "bridge" between the chemical and electrical energy of the fuel. In the process of oxidizing fuel into metabolites, the microbes in MFC gain energy by transferring the electrons produced in this process. However, in order to obtain a complete anode electron transfer mechanism, further investigation is needed.
In MFC, the substrate/fuel is the main factor affecting the entire process. Substrates used directly for MFC include pure cellulose and cellulose-rich feedstocks such as pre-treated corn stalks and seaweed. However, the complex structure of lignocellulose reduces the efficiency of power generation, resulting in lower electrical energy output. In order to improve the efficiency of MFC, the biomass needs to be pre-hydrolyzed to convert the biomass into soluble sugars, degraded phenolic compounds, acetic acid, furfural and 5-hydroxymethylfurfural (HMF) compounds. Prehydrolysis can produce different kinds of fermentable substrates from biomass. Glucose is one of the most important fuels extracted from biomass and is hydrolyzed from cellulose. Similarly, the hydrolysis of hemicellulose produces different pentose, hexose and acetic acid. These organic compounds show good power generation efficiency as carbon sources in MFC. Unlike the sugars in cellulose and hemicellulose, the phenols produced by lignin depolymerization have an inhibitory effect on microorganisms.
Although the concept of MFC has been around for a long time, industrial applications of MFC have been elusive. Limiting factors include the high cost, low yield, and limited durability of electrode materials and proton exchange membranes. The overall performance of an MFC depends on several factors, including: biolectation (i.e. reduced surface area due to blockage on the electrode surface), catalyst deactivation (if present), and excess biofilm growth. Excessive biofilm growth is due to the formation of non-conductive polymer fragments or dead cells, resulting in a decrease in the number of new generation biofilms. Other factors that lead to low MFC efficiency include electrocatalyst deactivation, fuel penetration from the anode chamber to the cathode chamber and vice versa, resulting in biofilm deactivation and mixed potential (i.e., system short circuit).
- ABB
- General Electric
- EMERSON
- Honeywell
- HIMA
- ALSTOM
- Rolls-Royce
- MOTOROLA
- Rockwell
- Siemens
- Woodward
- YOKOGAWA
- FOXBORO
- KOLLMORGEN
- MOOG
- KB
- YAMAHA
- BENDER
- TEKTRONIX
- Westinghouse
- AMAT
- AB
- XYCOM
- Yaskawa
- B&R
- Schneider
- Kongsberg
- NI
- WATLOW
- ProSoft
- SEW
- ADVANCED
- Reliance
- TRICONEX
- METSO
- MAN
- Advantest
- STUDER
- KONGSBERG
- DANAHER MOTION
- Bently
- Galil
- EATON
- MOLEX
- DEIF
- B&W
- ZYGO
- Aerotech
- DANFOSS
- Beijer
- Moxa
- Rexroth
- Johnson
- WAGO
- TOSHIBA
- BMCM
- SMC
- HITACHI
- HIRSCHMANN
- Application field
- XP POWER
- CTI
- TRICON
- STOBER
- Thinklogical
- Horner Automation
- Meggitt
- Fanuc
- Baldor
- SHINKAWA
- Other Brands




































































































































